
Stem cell therapy is a core topic in contemporary medical discussions. Donor-derived stem cells have been used in scientific research and clinical treatment for many years, and involve many types of cells, including mesenchymal stem cells (MSC), hematopoietic stem cells and stem cell derivatives. With the breakthrough of cell engineering technology, the field of stem cell therapy has ushered in revolutionary progress, greatly expanding the possibilities of treatment.
The advent of induced pluripotent stem cells (iPSC) has injected subversive innovative power into the progress of modern medicine (in 2007, Japanese scientist Nobu Yamanaka and China scientist Yu Junying also published the results of reprogramming human somatic cells into iPSC). This innovative achievement has released huge potential in multiple subject fields such as biology, pathology and regenerative medicine. Differentiation of target cells from iPSC has become a new trend in cell therapy research. Mesenchymal stem cells (MSC) have always been regarded as star cells in the field of regenerative medicine. With the development of iPSC technology, more and more experimental data have confirmed that compared with traditional MSC, iPSC-MSC not only has better proliferation ability, but also has a lower risk of age-related variability and heterogeneity. A series of experiments have further highlighted the significant advantages of iMSC.
01 Why has the issue of MSC heterogeneity been emphasized?
Mesenchymal stem cells (MSC) are a type of stem cells with self-renewal capabilities and multi-directional differentiation potential. They play a key role in immune regulation and tissue damage repair. In preclinical studies and clinical trials, MSC has shown potential for treating multiple diseases such as autoimmune diseases, tissue damaging diseases, and degenerative diseases. However, inconsistent clinical trial results suggest the complexity of MSC treatment.
With the deepening of research, it has been found that MSCs from different tissues such as umbilical cord, bone marrow, fat and pulp show heterogeneity in terms of surface marker expression, immunoregulatory functions, and proliferation and differentiation capabilities. These heterogeneity are closely related to the inconsistency of treatment effects. Therefore, addressing the heterogeneity of MSC from traditional sources is crucial to optimizing treatment options and improving treatment outcomes.

iMSC vs traditional source MSC
Dr. Yu Junying, one of the international pioneers of iPSC technology, once said that for cell therapy, the most important thing is how to obtain quality assurance and sufficient quantity of cell drugs at the clinical front end. Pluripotent stem cell technology is an ideal cell therapy platform and can be In vitro Mass production of stable quality cells solves the problems of cell quality and cell quantity that adult-derived cell therapies have always faced. As far as immunomaturing is concerned, super donor iPSC can be prepared, or universal pluripotent stem cells can be prepared through gene editing. So far, pluripotent stem cells, especially iPSC, are a technical platform that simultaneously solves the problems of cell quality, cell quantity and immune matching. Therefore, in terms of drug quality, iPSC-derived cell therapy products have very strong drug properties.
02 The latest experimental results of iMSC
����iPSC-MSC promotes muscle regeneration and avoids fibrosis
The study compared the therapeutic effects of adipose derived MSCs, bone marrow-derived MSCs, and gene-free iPSC-derived MSCs (XF-iMSC) in Ullrich Congenital Muscular Dystrophy (UCMD) mice. In the transplantation experiment of Col6a1-KO/NSG mice, the XF-iMSC transplantation group had significantly enhanced muscle fiber regeneration ability compared with other groups 1 week after transplantation (Figure 1). Twelve weeks after transplantation, the diameter of muscle fibers in the XF-iMSC transplantation group was larger than that in the other groups, and no fibrosis was induced (Figure 2). Similarly, in in vitro co-culture experiments, it was found that XF-iMSC promoted the fusion and differentiation of Col6a1-KO/NSG mouse-derived muscle stem cells (MuSC) more effectively than other primary MSC sources.
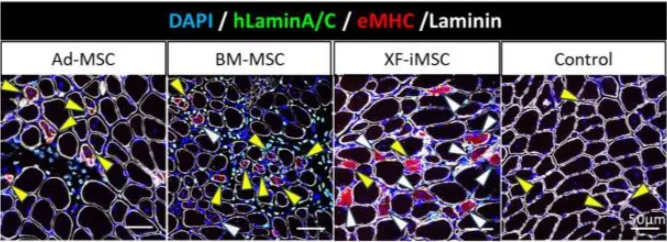
Figure 1 Cross-section of TA tibialis anterior muscle one week after transplantation
In the XF-iMSC transplantation group, the number (white arrows) and diameter of multinucleated regenerated muscle fibers increased; in the Ad-MSC and BM-MSC transplantation groups, although some multinucleated muscle fibers were observed (white arrows), the majority of regenerated muscle fibers were still mononuclear muscle fibers (yellow arrows)
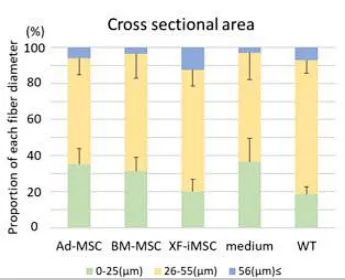
Figure 2 Proportion of muscle fibers with different diameters 12 weeks after transplantation
����iPSC-MSC derived from human urine cells has low immunogenicity and unique immunomodulatory properties
The study induced the differentiation of iPSC derived from human urine cell reprogramming into MSC (iMSC), and evaluated its immunogenicity, immunoregulatory ability and colonization in vivo. Compared with umbilical cord derived MSC (UCMSC), iMSC showed stronger proliferation, higher levels of regenerative gene expression, and lower immunogenicity, and enhanced its ability to resist apoptosis induced by allogeneic peripheral blood mononuclear cells (PBMC) and natural killer cell lines (NK-92)(Figure 3). In addition, compared to UCMSC, iMSCs have a reduced ability to inhibit T cell proliferation and activation. Transcriptomic analysis further revealed reduced expression of immune regulatory factors in iMSC. After infusion into a mouse model, iMSC colonized the lungs, liver, and spleen and demonstrated the ability to migrate to tumor tissue (Figure 4).
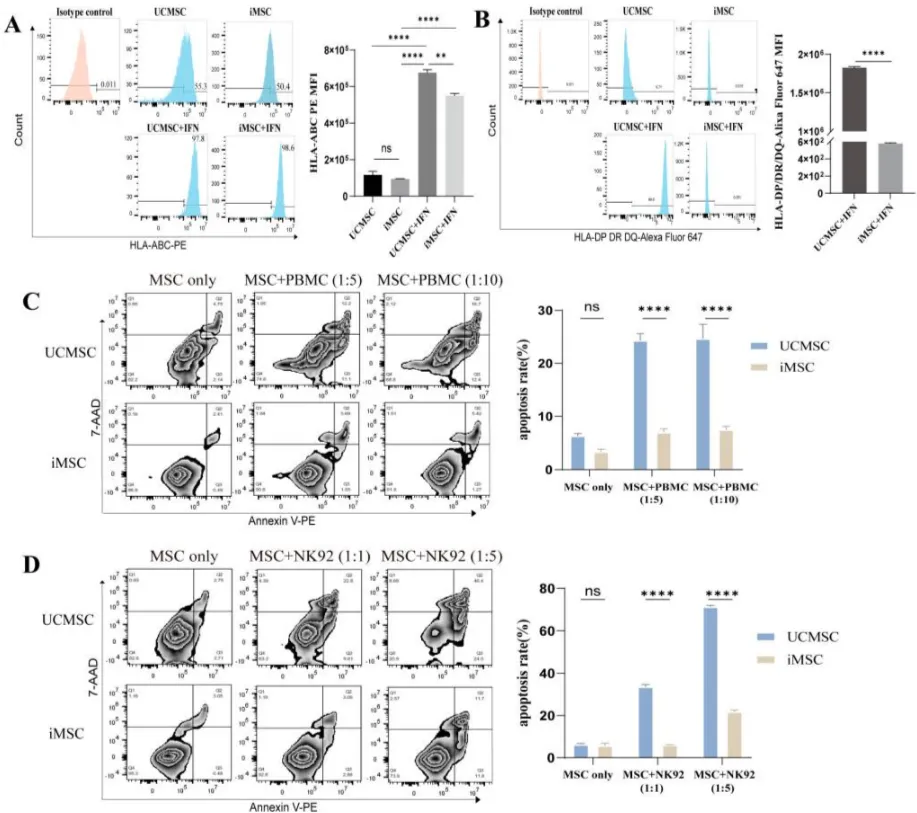
Figure 3 Low immunogenicity of iMSC

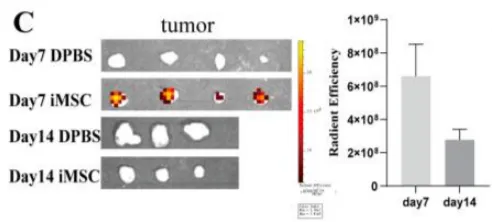
Figure 4 Colonization distribution of iMSC in mice
����iPSC-MSC lowers blood pressure and reduces target organ inflammation
To investigate the therapeutic effect of hiPSC-MSC (iMSC) in a rat model of spontaneous hypertension (SHR). iMSC significantly reduced blood pressure and reduced target organ inflammation in SHR rats (Figure 5). In addition, the blood pressure lowering effect of iMSC and human bone marrow-derived MSC in SHR was compared, and it was found that there were significant differences in the changes of systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean blood pressure (MBP) between the two groups. iMSC has a stronger blood pressure lowering effect than bone marrow-derived MSC (Figure 6). Cell tracking experiments showed that iMSC accumulates in the spleen of SHR, close to the splenic nerves, which may play a therapeutic role by affecting the splenic nerves and regulating ChAT+ cells. (iMSC releases large amounts of glutamate-activates the sympathetic nerves of the spleen-increases the release of norepinephrine (NE)-increases the number of choline acetyltransferase-positive (ChAT+) cells in the spleen-ChAT+ cells produce the vasodilator acetylcholine (ACh), which lowers blood pressure and reduces inflammation in target organs)
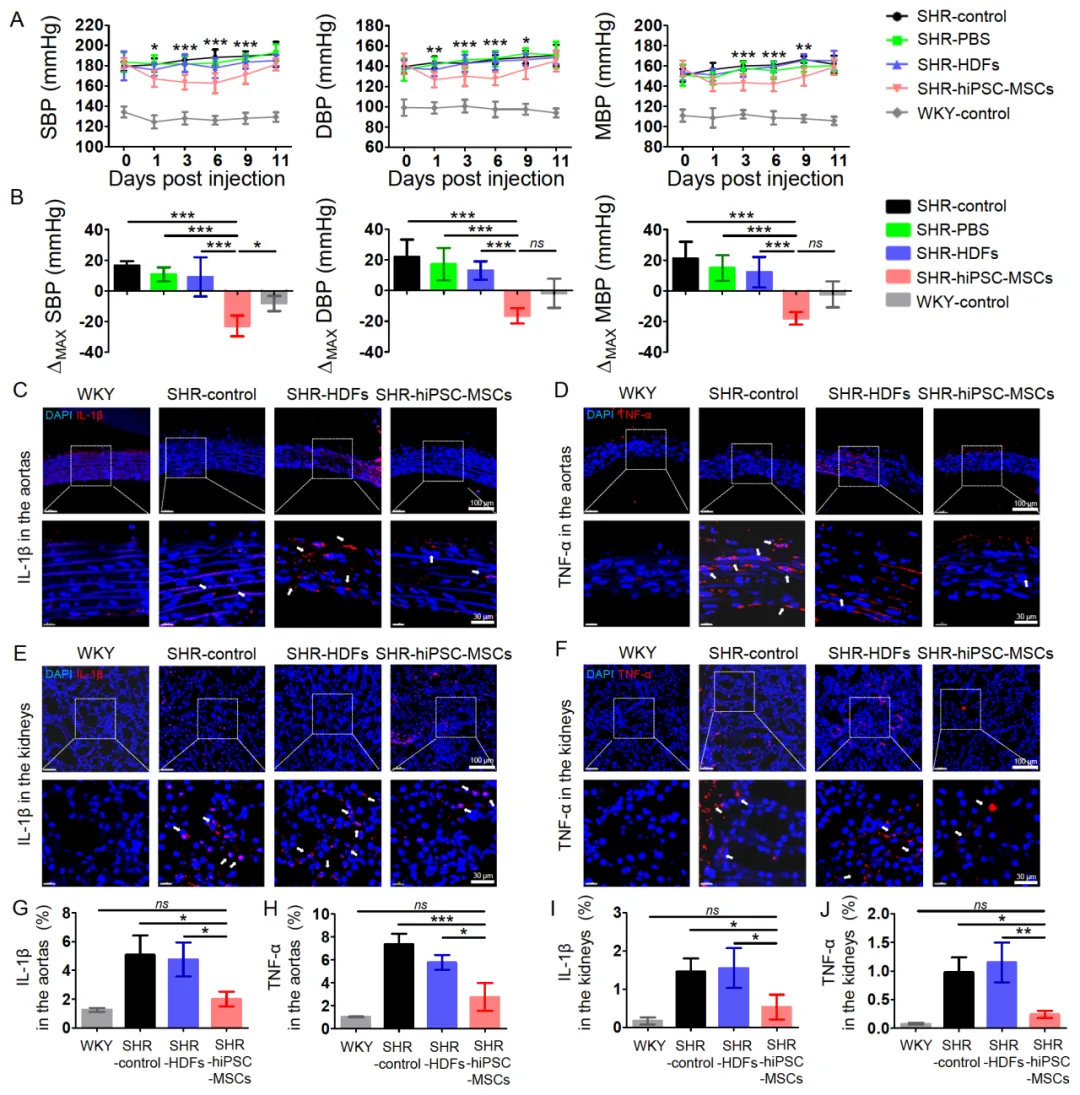
Figure 5 HiPSC-MSC alleviates hypertension and target organ inflammation in SHR rats
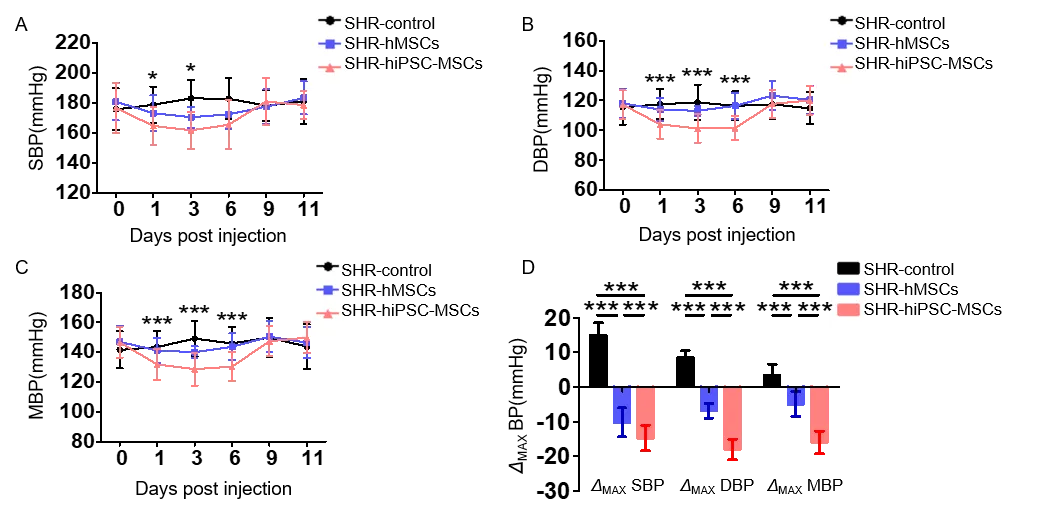
Figure 6 Comparison of blood pressure lowering effects of iMSC versus bone marrow-derived MSC
03 Clinical stage iMSC products
Currently, the iMSC products that have entered the clinical stage include Cynata from Australia. The three products CYP001, CYP002 and CYP004 are all in the clinical stage. Among them, CYP004 is in clinical stage III for the treatment of knee osteoarthritis, making it the fastest-progressing product in the world for iPSC-derived cell therapy. In addition, the NCR100 product traced by Zhongsheng in China is the first and only iMSC product approved for clinical use in China.
|
Products |
Company |
Indications |
Stage |
|
CYP001 |
Cynata |
Graft versus host disease (GVHD) |
II |
|
CYP002 |
Cynata |
Severe lower limb ischemia (CLI) |
II |
|
CYP004 |
Cynata |
Knee osteoarthritis (KOA) |
III |
|
NCR100 |
中盛溯源 |
Knee osteoarthritis (KOA) |
I/II |
04 Summary
MSC has shown great potential in treating a variety of diseases, and more than 10 adult-derived MSC products have been approved for marketing around the world. These products are mainly based on the immunomodulatory and tissue repair properties of MSCs and are used to treat diseases such as graft-versus-host disease, knee osteoarthritis, Crohn's disease and severe lower limb ischemia. However, the inherent heterogeneity of adult-derived MSCs further affects the consistency of clinical trial results. iPSC-MSC, derived from the same monoclonal iPSC seed cell, provides a way to address heterogeneity and achieve mass production issues. In the future, with the continuous optimization of iPSC technology, iPSC-MSC products are expected to further improve the safety and effectiveness of treatment and promote innovation and development in the field of stem cell therapy.